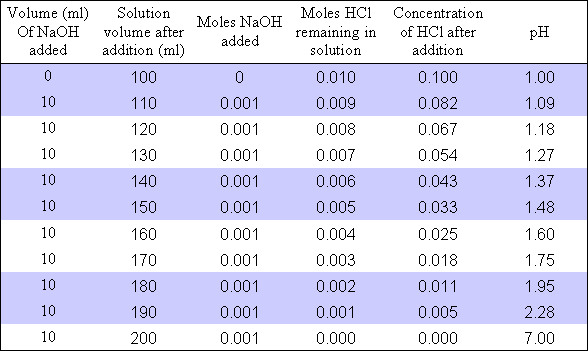
Ph Of Buffer Solution After Adding Hcl
Buffer problems 1 a buffer is prepared by adding 060 moles of hc2h3o2 and 20 moles of nac2h3o2 to enough water to make 10 dm 3 of solution.

Ph of buffer solution after adding hcl. Commercially prepared ph 686 standard buffer solution ph of 686 at 25 degrees c o. Ph buffer 686 is a mixture of potassium and sodium phosphates. Examples of calculation of buffer ph change after addition of strong acidbase. This phenol solution product no.
P4557 includes a separate bottle of equilibration buffer product no. B5658 to adjust the phenol phase to ph 7902. If we add a strong acid or strong base to water the ph will change dramatically. For instance adding a strong acid such as hcl to water results in the reaction hcl.
During optimization of an extraction procedure we found out that salt concentration has a significant effect on the ph value of buffer solutions. Ph measurement troubleshooting problem. The ph probe response is slow tends to drift and results are not reproducible. This may be caused by one of the.
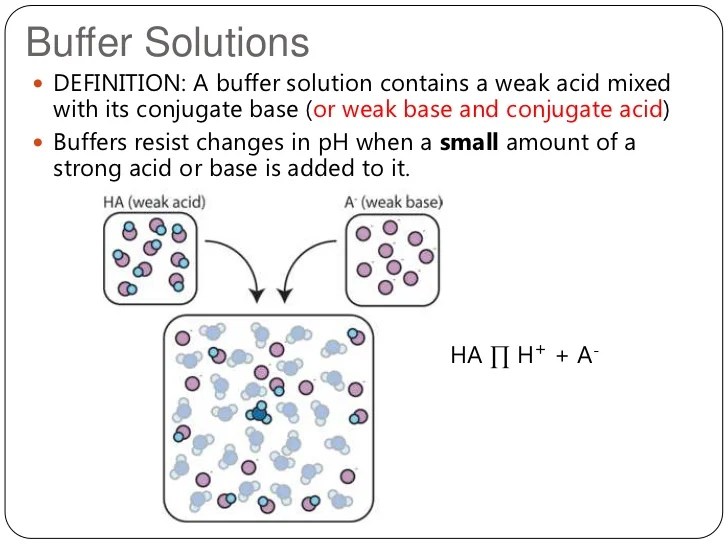
This tutorial describes the concept of the effectiveness of a buffers ability to offset the effects of adding a strong acid or base on the ph of a system.